Gastroenterology
Comprehensive GI Pathology Services


Gastroenterologists & Primary Care Providers
QDx provides a full range of GI tests and pathology services focused on supporting your screening, diagnostic and case management strategies. Our pathologists are ready to collaborate with you on identifying the right pathway forward for successful clinical outcomes.
Our GI pathologists provide expert interpretation of a full range of cases that focus on the diagnosis and characterization of neoplastic and non-neoplastic diseases with special emphasis on chronic inflammatory diseases and tumors in the GI tract, pancreas and liver.
- Fellowship Trained GI pathologists:
QDx staff GI pathologists are fellowship trained in GI and liver pathology and have special interest in Pediatric GI pathology. - Personal Consultation:
Our pathology staff provides personal consultation with the ordering physician on unusual cases upon request. - Interpretive Reports:
Images and graphic reporting elements are included in the final report which aids in the understanding of the findings.
QDx offers a variety of diagnostic assessments including testing for viruses, bacteria and parasites that can cause gastrointestinal infections, disease and complications. The laboratory employs advanced PCR methods and instrumentation to provide you with rapid, accurate and precise results.
GI PATHOGEN PANEL
GI Pathogen Panel is based on multiplexed nucleic acid detection and is intended for the simultaneous qualitative detection and identification of multiple viral, bacterial and parasitic nucleic acids in human stool samples. This includes Cary-Blair media from individuals with signs and symptoms of gastrointestinal infection.
Turn Around Time – 48 hours.
CLOSTRIDIUM DIFFICILE TOXINS A AND B
Clostridium difficile infection involves a range of clinical presentations from mild diarrhea to life-threatening pseudomembranous colitis and megacolon. Moderate to severe CDI cases require early identification for better outcomes and decreased mortality. Especially among the elderly.
Turn Around Time – 24 hours.
HELICOBACTER PYLORI STOOL ANTIGEN
H. Pylori is responsible for 90% of duodenal ulcers and 80% of gastric ulcers. It is also associated with a 2-3 fold increased risk of gastric cancer and mucosal associated-type (MALT) lymphoma.
H. pylori stool antigen is a qualitative test that detects Helicobacter pylori specific antigen. It is intended for use with human fecal specimens to aid in the diagnosis of H. pylori infection and to demonstrate loss of H. pylori antigen following treatment.
Turn Around Time – 48 hours.
GI Pathogen Panel
BACTERIA
- Campylobacter (C. jejuni, C. coli)
- Clostridium difficile, Toxin A/B (Reflex to C. diff. toxin A/B EIA)
- Diarrheagenic E. coli/Shigella (E. coli 0157, EAEC, ETEC, STEC, EIEC)
- Salmonella
- Vibrio spp. (V. vulnificus/V. cholera, Vibrio parahaemolyticus)
- Yersinia enterocolitica
PARASITES
Entamoeba histolytica
Giardia lamblia
VIRUSES
Adenovirus F40/41
Norovirus GI/GII
Rotavirus A
Clostridium Difficile Toxins A and B
The QDx Clostridium Difficile assay when combined with QDx Lactoferrin testing provides both an indication of the presence of stool toxins and the degree of intestinal inflammation as an indicator of disease severity in CDI patients.
QDx offers Clostridium Difficile analysis based on an enzyme immunoassay used for the detection of toxins A and B produced by toxigenic strains of Clostridium difficile. The assay can be used to detect toxins A and B in fecal specimens from persons suspected of having C. difficile disease.
Helicobacter Pylori Stool Antigen
Using the Gen-Probe APTIMA COMBO 2 Assay from Chlamydia trachomatis and Neisseria gonorrheae, QDx Pathothology can qualitatively detect and differentiate RNA to provide accurate identification of infection using the Thin Prep vial. The Assay delivers more sensitivity than a culture, allowing rapid and reliable test results.
SERUM ANTIBODY TESTING FOR H. PYLORI IS NOT ACCURATE FOR CURRENT INFECTIONS
- Serum antibody tests detect antibodies from both current and prior infections
- No information is provided on whether an infection is the source of symptoms
- Clinician collected PreservCyt Solution liquid Pap specimens
- Antibodies are detected with this method years after an infection has resolved
STOOL ANTIGEN TESTING IS COMPARABLE TO ENDOSCOPY / HISTOLOGY FOR IDENTIFYING CURRENT H. PYLORI INFECTIONS
- Stool antigen testing is a widely accepted non-invasive frontline tool for identifying patients with an H. pylori infection
- Stool antigen testing reflects infection throughout the stomach and duodenum, an advantage as sites of infection in the stomach are often patchy and require multiple biopsies to ensure detection and estimate extent of disease
RECOMMENDATIONS FROM THE 2017 ACG GUIDELINES FOR H. PYLORI TESTING
- H. pylori testing should be performed for all patients with active peptic ulcer disease, mucosa-associated lymphoma or early gastric cancer
- Non-endoscopic testing for H. pylori should be considered for dyspeptic patients under the age of 60
- All tested patients who are positive for H. pylori infections using tests recommended in guidelines such as stool antigen testing should be offered
- Due to high rates of antibiotic resistance, patients should be re-tested after treatment to confirm eradication of the organism
- The 2017 ACG Guidelines prefer testing methods such as stool antigen testing that detect current disease
Our stool testing services support the determination of the underlying causes of gastrointestinal illnesses (bacteria, parasites), as well as diseases, such as colitis or inflammatory bowel disease.
Fecal Fat
Fecal fat measures the amount of fat within a stool sample. Excess fat known as steatorrhea can be tested for this, ultimately aiding in the determination of malabsorption and/or digestive disease. We test for both the presence of neutral fats and split fats in stool. Neutral fats (e.g., monoglycerides, diglycerides, triglycerides) seen in excess is suggestive of steatorrhea, impaired synthesis or secretion of pancreatic enzymes or bile. An increase in split fats is suggestive of impaired absorption of nutrients. These neutral/split fats should be rare in normal stool samples.
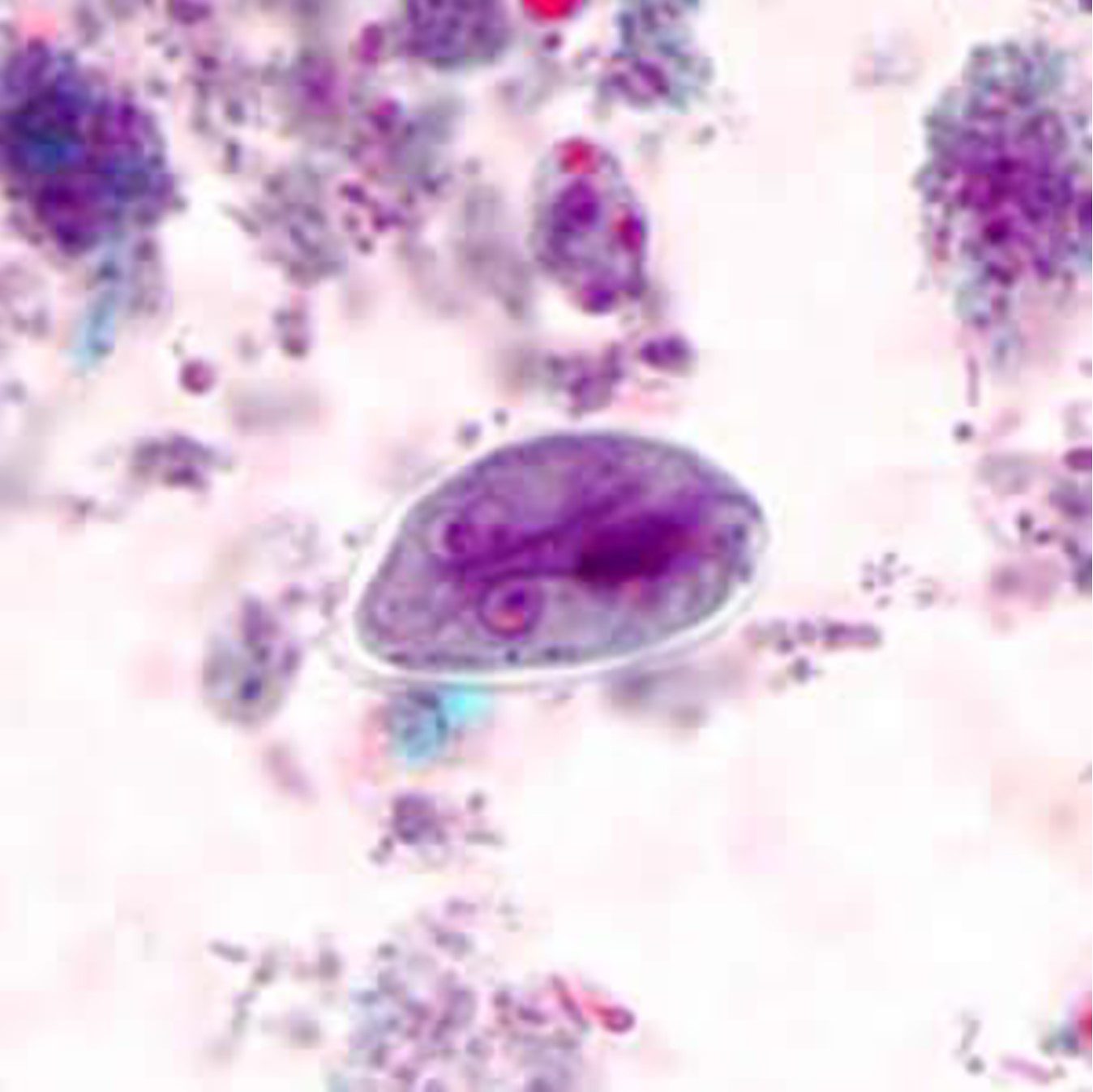
OVA AND PARASITES SCREENING TEST
The Ova and Parasite stool test is a microscopic exam to help diagnose the appearance of eggs, trophozoites and/or helminths found within stool. Parasitic infections often occur in the lower digestive tract, which may cause diarrhea.
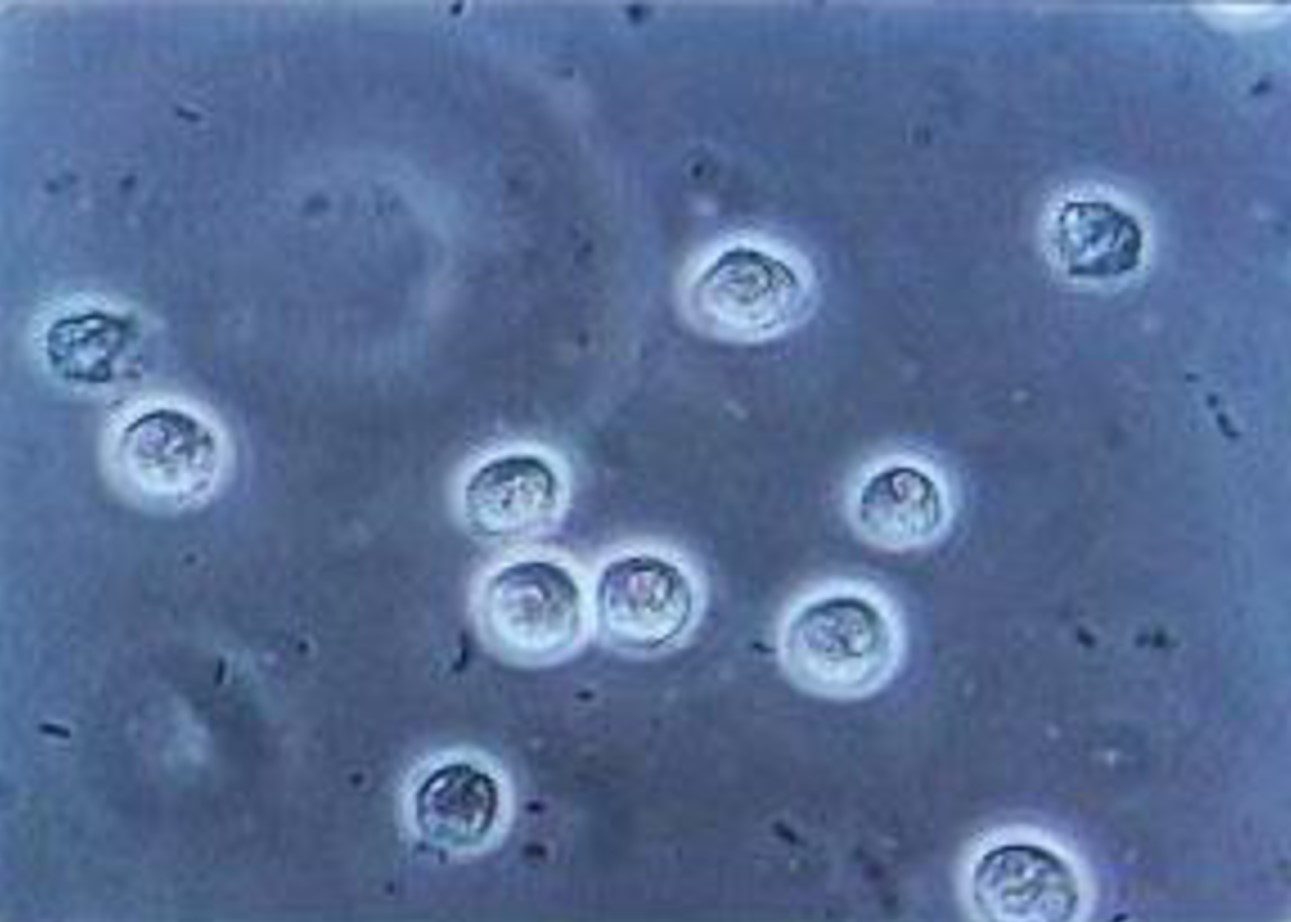
FECAL WHITE BLOOD CELL (WBC) STOOL TEST
White Blood Cell stool testing or Fecal Leukocyte testing help diagnose inflammatory diarrhea. This type of diarrhea may be an indication of an infection by bacteria, or the result of ulcerative colitis or inflammatory bowel disease.
Note: If ordering Calprotectin or Lactoferrin, Fecal WBC is of limited value and should not be ordered.
QDx offers several immunoassays to detect the presence of blood, bacteria or parasites, as well as other signs of diseases and disorders. Our laboratory utilizes advanced analytic methods and equipment including chemiluminescence immunoassays that improve detection sensitivity when compared to standard immunoassays.
CALPROTECTIN, FECAL
LACTOFERRIN, FECAL
PANCREATIC ELASTASE ELISA, FECAL
Calprotectin, Fecal
METHOD
Calprotectin is a chemiluminescent immunoassay for the quantitative determination of fecal calprotectin in extracted human stool samples.
CLINICAL USE
Elevated levels of fecal calprotectin, in conjunction with clinical findings and other laboratory tests, can aid in the diagnosis of inflammatory bowel disease (IBD) (ulcerative colitis and Crohn’s disease), and in the differentiation of IBD from irritable bowel syndrome (IBS).
Pancreatic Elastase ELISA, Fecal
METHOD
This test is ELISA monoclonal antibody test intended for the quantitative determination of pancreatic elastase (enzyme produced in the acinar cells of the pancreas that enhances the digestive process) in human stool samples.
CLINICAL USE
The test may be used as an aid in the diagnosis or exclusion of exocrine pancreatic insufficiency associated with chronic pancreatitis, cystic fibrosis, carcinoma of the pancreas, diabetes mellitus type 1 and other etiologies of pancreatic insufficiency. Pancreatic elastase is mainly bound to bile salts during intestinal passage and is not degraded. As a result, in human feces, it is 5-6 fold more concentrated than in pancreatic juice. The stool concentration reflects the secretory capacity of the pancreas. Studies have shown pancreatic elastase to be an important biomarker of exocrine pancreas function, and that it can also be used to define or exclude exocrine pancreatic insufficiency in cases of unexplained diarrhea, constipation, weight loss and food intolerances.
Lactoferrin, Fecal
METHOD
LACTOFERRIN SCAN® test is a quantitative test that measures the level of fecal lactoferrin released from leukocytes.
CLINICAL USE
Fecal lactoferrin testing can help to differentiate active IBD from IBS. It can also help to determine intestinal inflammation during CDI. Other intestinal ailments, including many gastrointestinal infections and colorectal cancer, often result in elevated levels of lactoferrin in fecal specimens. Therefore, when evaluating a patient, a clinical assessment must be considered along with test results.
LIMITATIONS
- Fecal samples from breast-fed infants should not be used with this assay. The test may not be appropriate in immunocompromised persons.
- The patient samples listed below should be excluded from use in the test:
- Patients with a history of HIV and/or Hepatitis B and C
- Patients with a history of infectious diarrhea (within 6 months)
- Patients receiving a colostomy and/or ileostomy within one month
Note: Lactoferrin will not be performed on watery diarrhea samples due to limited specificity and sensitivity.
Clinical Management Report
Upon request, QDx provides your practice with clinical management reports that summarize aggregate test result data to support your quality assurance process. Contact your QDx representative to learn more.