Urology
Innovative reporting for prostate and bladder cancer


Partners in Pathology with Patient-Centric Care
QDx provides a full range of tests and pathology services focused on supporting your screening, diagnostic and case management strategy. Our pathologists are ready to collaborate with you on identifying the right pathway forward for successful clinical outcomes.
Cystoscopy examination of the bladder, and often urine cytology, are standard care for patients >40 years of age and presenting with hematuria.4 QDx provides the pathology and testing services to support screening, recurrence monitoring and detection of bladder cancer.
FISH BLADDER CANCER SCREENING
Patients with hematuria and suspected of having bladder cancer should be screened with FISH test as a complimentary procedure to a cytopathology examination. QDx utilizes the FDA approved Abbott UroVysion test kit to detect bladder cancer in urine. The fluorescence in situ hybridization assay utilizes fluorescently labeled DNA probes. The method quantifies aneuploidy chromosomes 3, 7, 17, and the loss of the 9p21 locus via fluorescence in situ hybridization (FISH) in urine samples.
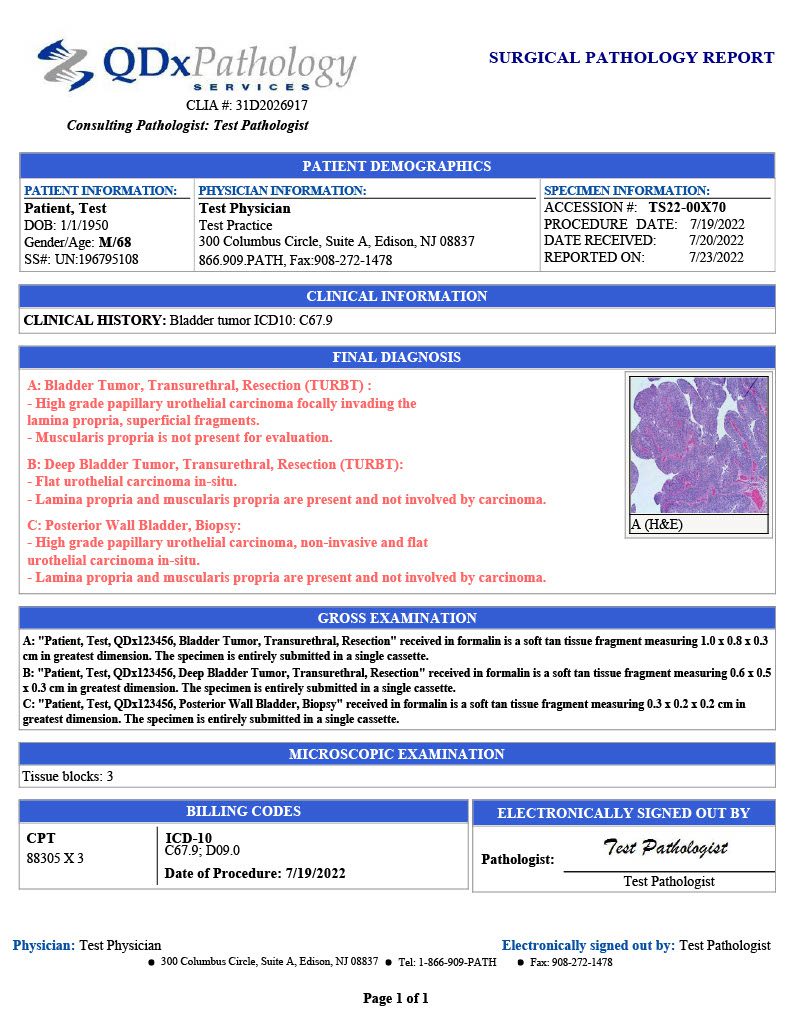
BLADDER CANCER PATHOLOGY EXAMINATION
QDx Pathologists provide pathology examination and interpretation of bladder cells utilizing special stains and select IHC stains. QDx employs pathologists with uropathology specialization and training. When necessary, our pathologists provide personalized consultations to the referring provider.
Diagnostic services cover a variety of bladder cancers including:
- Urothelial Carcinoma (transitional cell carcinoma) TCC
- Squamous Cell Carcinoma
- Adenocarcinoma
- Small Cell Carcinoma
- Sarcoma
- Carcinoma in Situ (CIS)
- Metastatic Carcinoma
4.Grossfeld GD, Wolf JS, Litwin MS, et al. Asymptomatic Microscopic Hematuria in Adults: summary of AUA best practice policy recommendations. Am Fam Phys. 2001;63(6):1145-1154
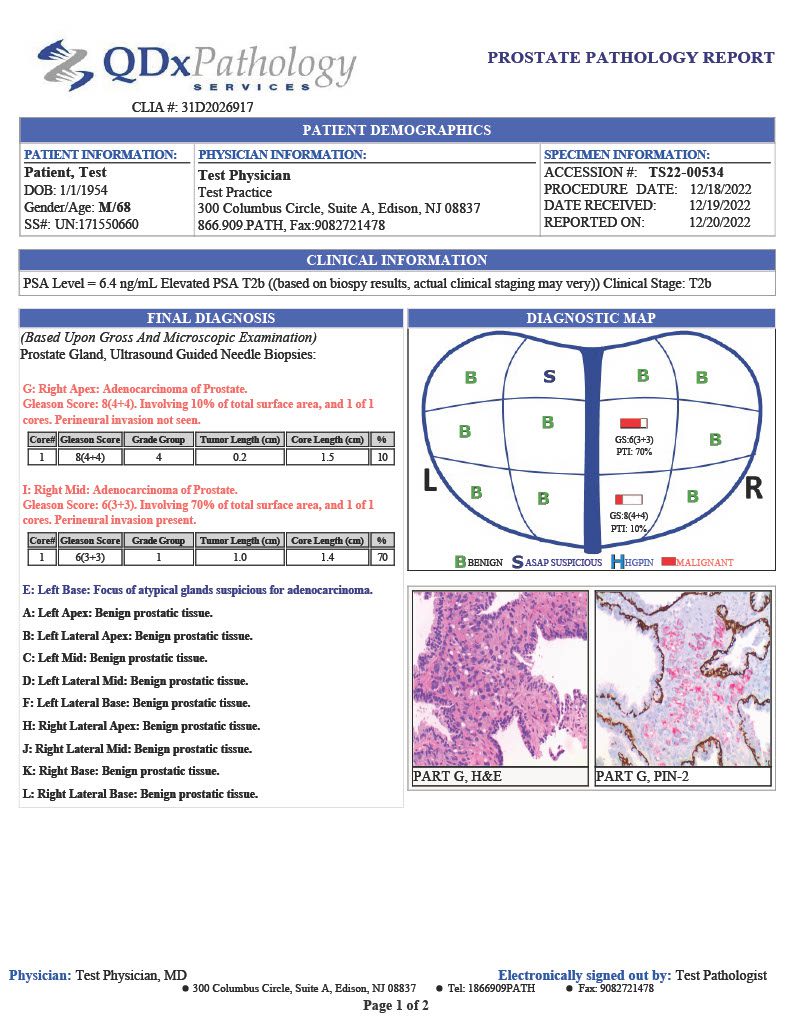
Prostate cancer is the second leading cancer diagnosis in men with 268,000 new cases diagnosed each in the United States.1 The QDx pathology team provides expert evaluation to inform treatment strategy.
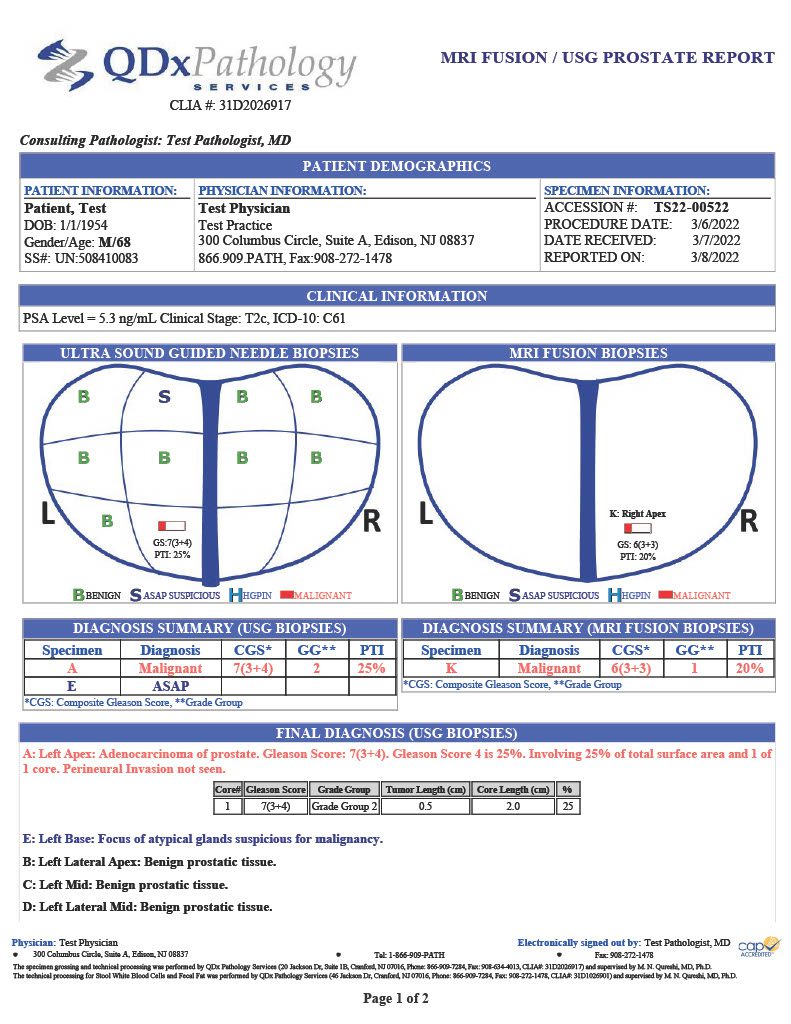
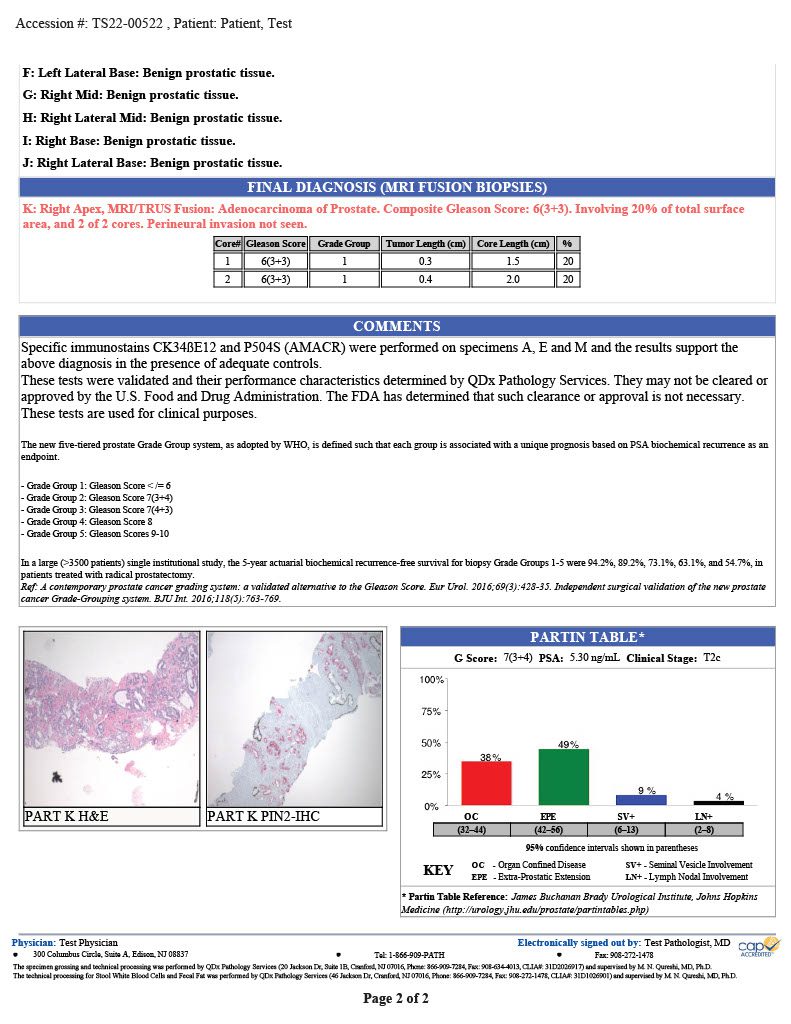
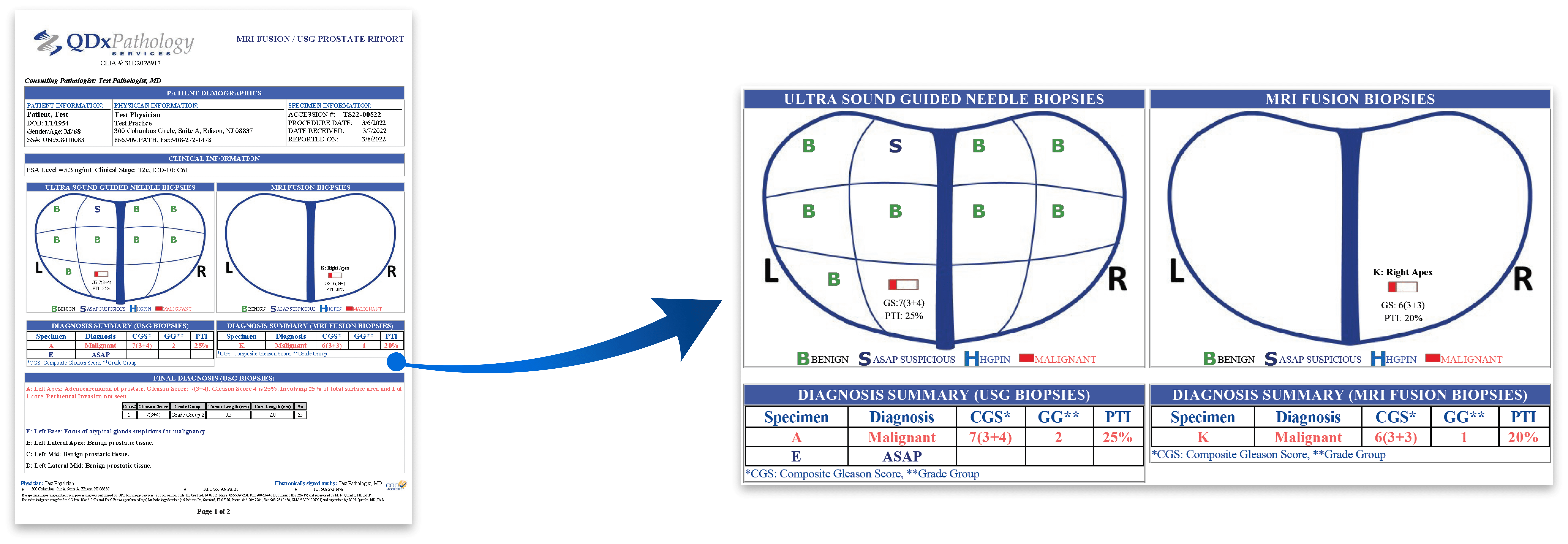
QDx pathologists employ H&E and IHC stains to support their evaluation of prostate tumors. Final reports include pathologist interpretation, Gleason grading score, Partin table and graphical representation of the tumor location.
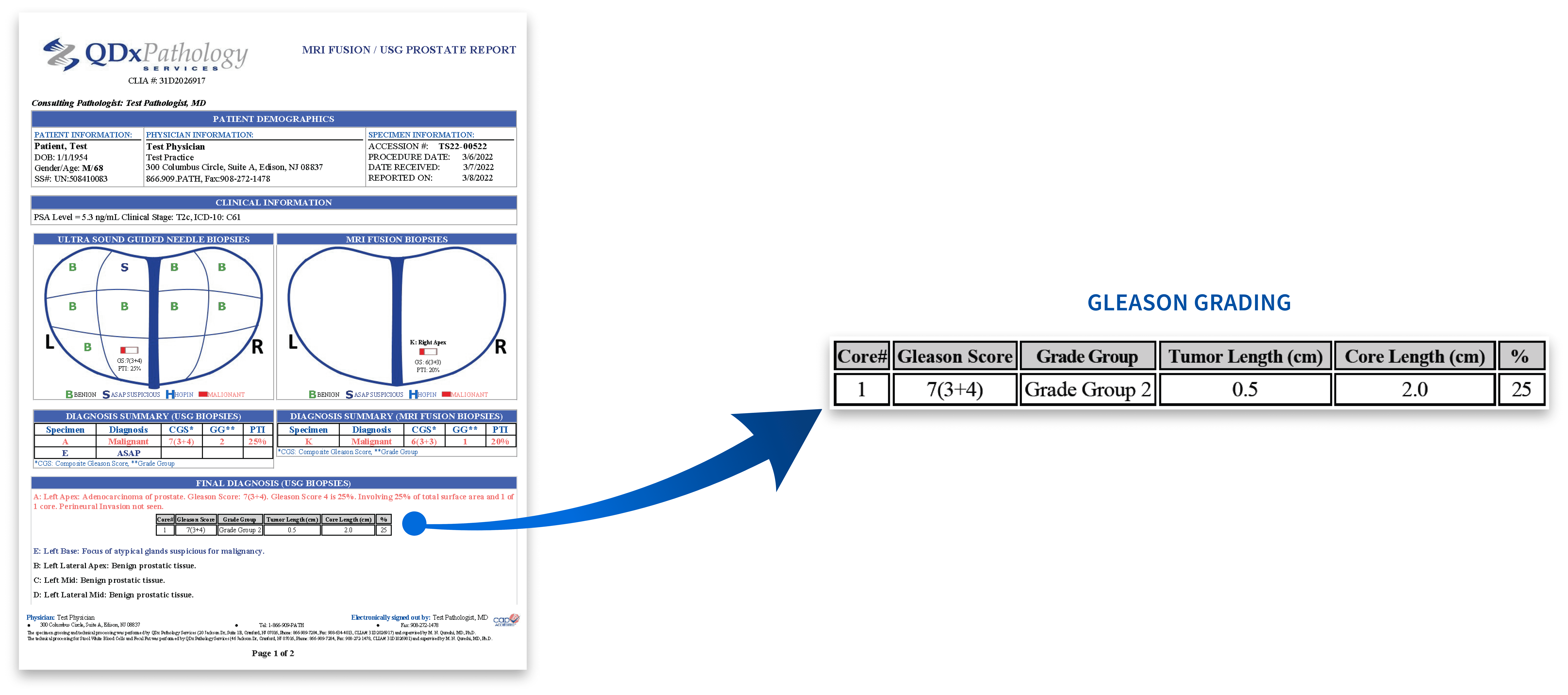
GLEASON GRADING
QDx pathologists determine and report a Gleason score for each tumor by evaluating and grading the tumor in comparison with normal prostate glands. Higher scores reflect increasingly complicated glandular proliferations.
SCORING METHODOLOGY
The pathologist grades each sample of prostate cancer cells from 3 to 5 based on how quickly they are likely to grow or how aggressive the cells look. The scoring outline and report examples are listed below.
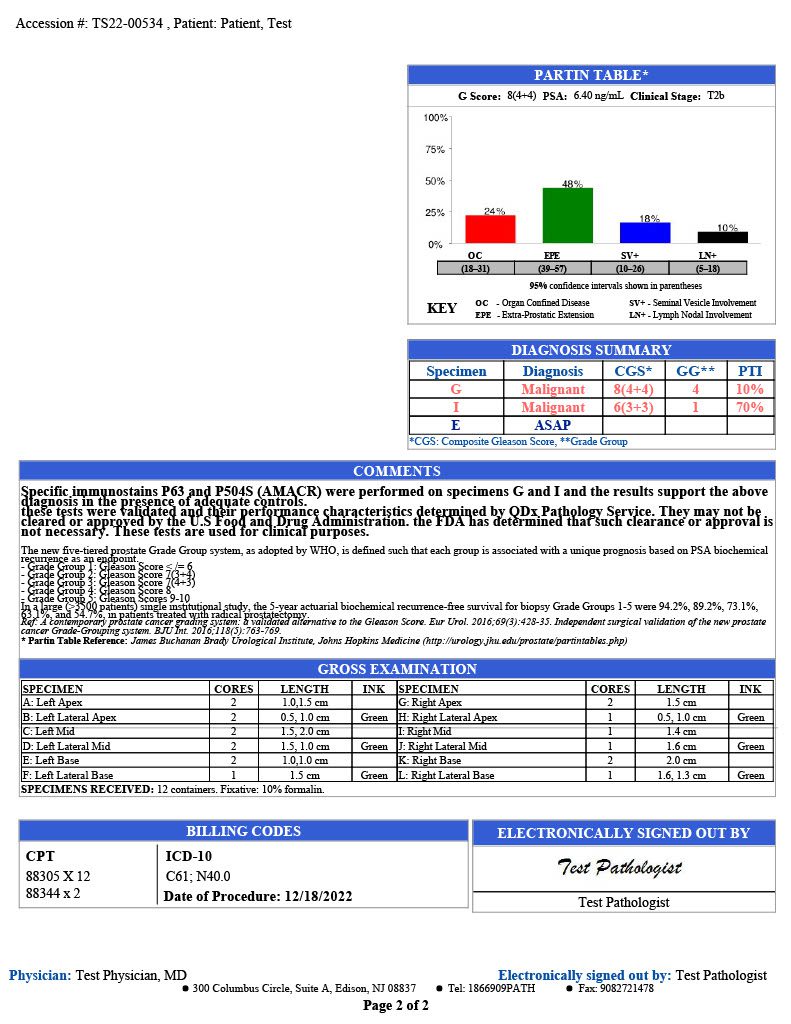
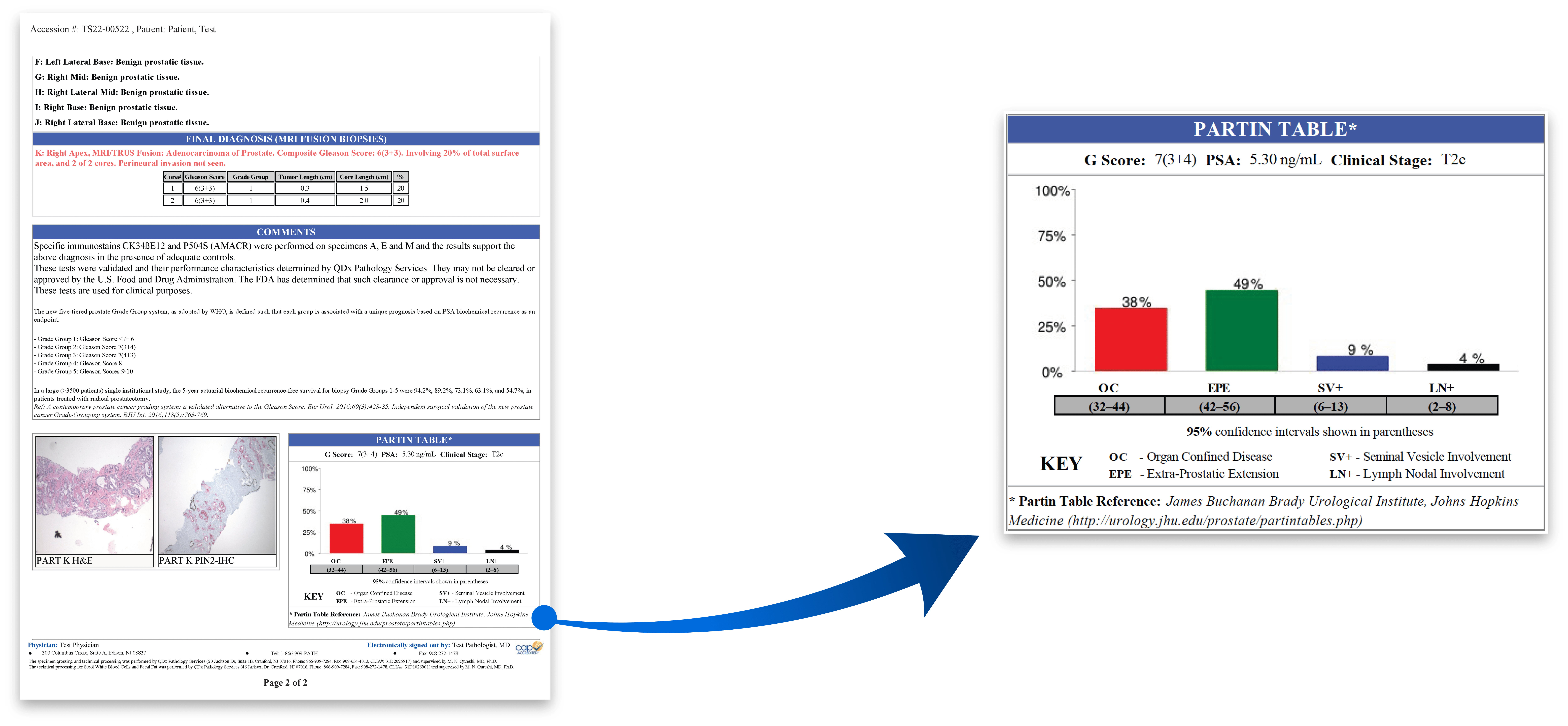
PARTIN TABLE
QDx includes a Partin table in its prostate pathology report which includes the clinical features of prostate cancer such as a Gleason score, serum PSA, and clinical stage to predict whether the tumor will be confined to the prostate. The tables are based on the accumulated experience of urologists performing radical prostatectomy at the James Buchanan Brady Urological Institute.
GRAPHIC REPORTING
QDx includes a graphic representation of the biopsy sample locations with the associated interpretation of each part including ultrasound guided needle biopsies and MRI Fusion biopsies.
- American Cancer Society
Scoring Methodology
Gleason Grading Guide
| Gleason Score | Grade Group | What It Means |
|---|---|---|
| Gleason score 6 (or 3+3=6) | Grade Group 1 | The cells look similar to normal prostate cells. The cancer is likely to grow very slowly, if at all. |
| Gleason Score 7 (or 3+4=7) | Grade Group 2 | Most cells still look similar to normal prostate cells. The cancer is likely to grow slowly. |
| Gleason Score 7 (or 4+3=7) | Grade Group 3 | The cells look less like normal cells. The cancer is likely to grow at a moderate rate. |
| Gleason Score 8 (or 4+4=8) | Grade group 4 | Some cells look abnormal. The cancer might grow quickly or at a moderate rate. |
| Gleason Score 9 (or 4+5=9, 5+4=9, or 5+5=10) | Grade Group 5 | The cells look very abnormal. The cancer is likely to grow quickly. |
Gleason score 6 (or 3+3=6) - Grade Group 1
The cells look similar to normal prostate cells. The cancer is likely to grow very slowly, if at all.
Gleason Score 7 (or 3+4=7) - Grade Group 2
Most cells still look similar to normal prostate cells. The cancer is likely to grow slowly.
Gleason Score 7 (or 4+3=7) - Grade Group 3
The cells look less like normal cells. The cancer is likely to grow at a moderate rate
Gleason Score 8 (or 4+4=8) - Grade group 4
Some cells look abnormal. The cancer might grow quickly or at a moderate rate.
Gleason Score 9 (or 4+5=9, 5+4=9, or 5+5=10) - Grade Group 5
The cells look very abnormal. The cancer is likely to grow quickly.
STI PANELS
Chlamydia trachomatis infections are one of the most common sexually transmitted infections worldwide. In the United States alone, an estimated 1,598,354 (497 cases per 100,000 population) new cases of CT infections were reported to the Centers for Disease Control in 2016. Using the Gen-Probe APTIMA COMBO 2 Assay for Chlamydia trachomatis and Neisseria gonorrhoeae, QDx Pathology can qualitatively detect and differentiate RNA to provide accurate identification of infection using the Thin Prep vial. The assay is more detailed and specific than a culture, allowing for rapid and reliable test results.
Turn Around Time: 48 hours
HPV TESTING
QDx HPV testing provides detection of human papillomavirus E6/E7 mRNA, histological localization of HPV within the tissue, and differentiation of low-risk vs. high-risk subtypes in formalin-fixed paraffin-embedded tissues.
Turn Around Time: 48 hours
STI Test Details
CT/NG
Chlamydia trachomatis
Neisseria gonorrhoeae
TAT: 3-4 Days
HPV with Reflex to Genotyping for 16, 18/45 if HPV+
Tests for high-risk human papillomavirus (HPV) types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) without differentiation and if positive, reflexes to genotyping for HPV types 16 and 18,45; type 18 cannot be differentiated from type 45.
TAT: 3-4 Days
HSV 1&2
Herpes Simplex Virus 1 (HSV1)
Herpes Simplex Virus 2 (HSV2)
TAT: 3-4 Days
Mycoplasma genitalium
TAT: 2-4 Days
Trichomonas vaginalis
TAT: 2-4 Days
HPV Testing
HPV w/ Genotype 16,18,45
- HPV DNA Genotyping 16,18,45
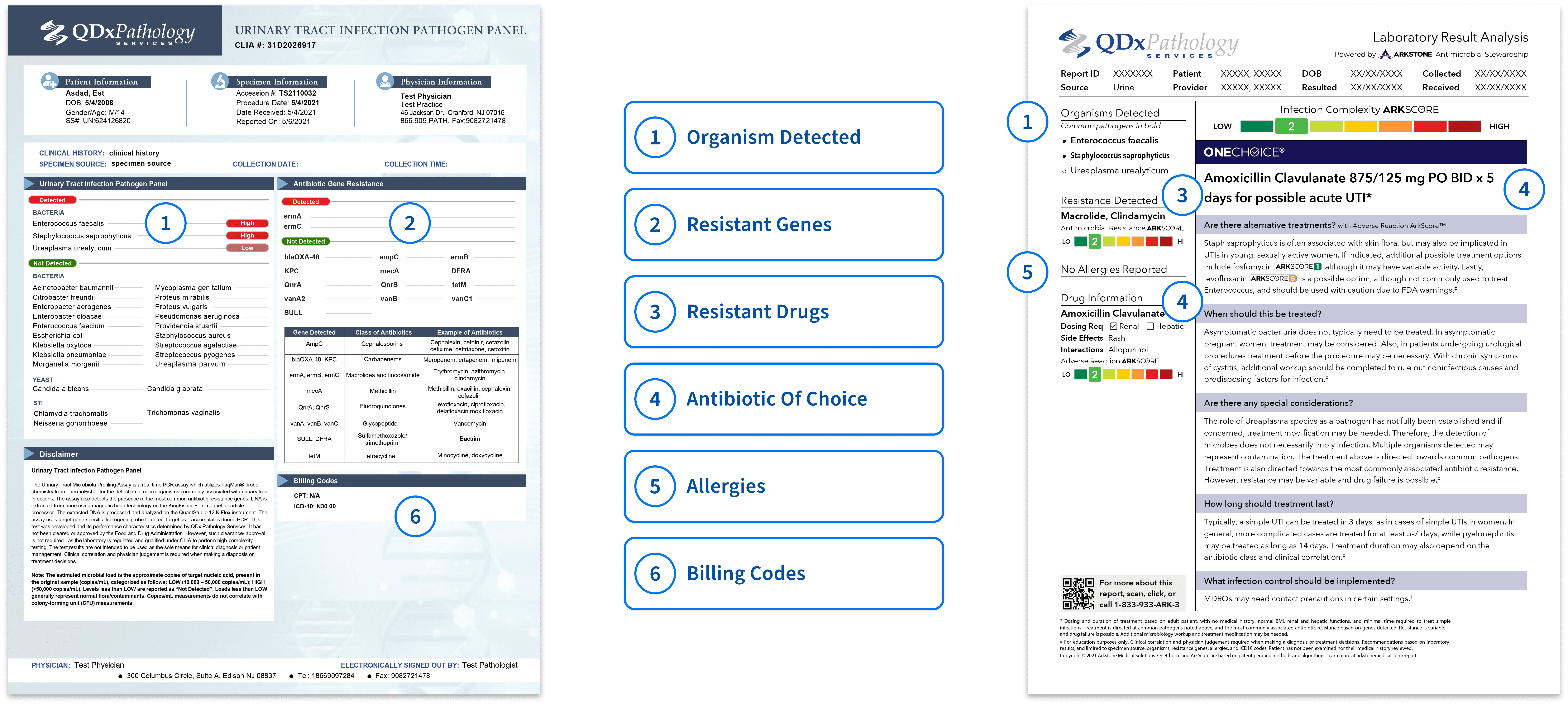
QDx UTI pathogen panel is a PCR assay for the detection of microorganisms commonly associated with urinary tract infections. The assay also detects the presence of the most common antibiotic resistant genes.
- Turn Around Time: 24-48 hours
PATHOGENS
ANTIBIOTIC RESISTANT REPORTING
UTI PATHOGEN PANEL REPORT
The UTI Pathogen panel report is presented in an easy-to-read format with color keys indicating if any pathogen resistant genes are detected and provides the recommended antibiotic.
Pathogens
Bacteria
Morganella morganii
Ureaplasma aparvum
Acinetobacter Baumannii
Mycoplasma genitalium
Ureaplasma urealyticum
Citrobacter freundii
Proteus mirabillis
Enterobacter cloacae
Providencia stuartii
Enterobacter aerogenes
Streptococcus agalactiae
Proteus vulgaris
Enterococcus faecalis
Pseudomonas aeruginosa
Enterococcus faecium
Staphylococcus aureus
Escherichia coli
Staphylococcus saprophyticus
Klebsiella oxytoca
Streptococcus pyogenes
Klebsiella pneumoniae
Yeast
Candida Albicans
Candida glabrata
STI
Chlamydia trachomatis
Neisseria gonorrhoeae
Trichomonas vaginalis
Antibiotic Resistant Gene Key
| Gene Detected | Class of Antibiotics | Example of Antibiotics |
|---|---|---|
| AmpC | Cephalosporins | Cephalexin, cefdinir, cefazolin cefixime, ceftriaxone, cefoxitin |
| blaOXA-48, KPC | Carbapenems | Meropenem, ertapenem, imipenem |
| ErmA, ErmB, ErmC | Macrolides and lincosamide | Erythromycin, azithromycin, clindamycin |
| mecA | Methicillin | Methicillin, oxacillin, cephalexin, cefazolin |
| QnrA, QnrS | Fluoroquinolones | Levofloxacin, ciprofloxacin, delafloxacin moxifloxacin |
| VanA, VanB, VanC | Glycopeptide | Vancomycin |
| SULL, DFRA | Sulfamethoxazole/ trimethoprim | Bactrim |
| tetM | Tetracycline | Minocycline, doxycycline |
AmpC
Class of Antibiotics:
Cephalosporins
Example of Antibiotics:
Cephalexin, cefdinir, cefazolin cefixime, ceftriaxone, cefoxitin
blaOXA-48, KPC
Class of Antibiotics:
Carbapenems
Example of Antibiotics:
Meropenem, ertapenem, imipenem
ErmA, ErmB, ErmC
Class of Antibiotics:
Macrolides and lincosamide
Example of Antibiotics:
Erythromycin, azithromycin, clindamycin
mecA
Class of Antibiotics:
Methicillin
Example of Antibiotics:
Methicillin, oxacillin, cephalexin, cefazolin
QnrA, QnrS
Class of Antibiotics:
Fluoroquinolones
Example of Antibiotics:
Levofloxacin, ciprofloxacin, delafloxacin moxifloxacin
VanA, VanB, VanC
Class of Antibiotics:
Glycopeptide
Example of Antibiotics:
Vancomycin
SULL, DFRA
Class of Antibiotics:
Sulfamethoxazole/ trimethoprim
Example of Antibiotics:
Bactrim
tetM
Class of Antibiotics:
Tetracycline
Example of Antibiotics:
Minocycline, doxycycline
Clinical Management Report
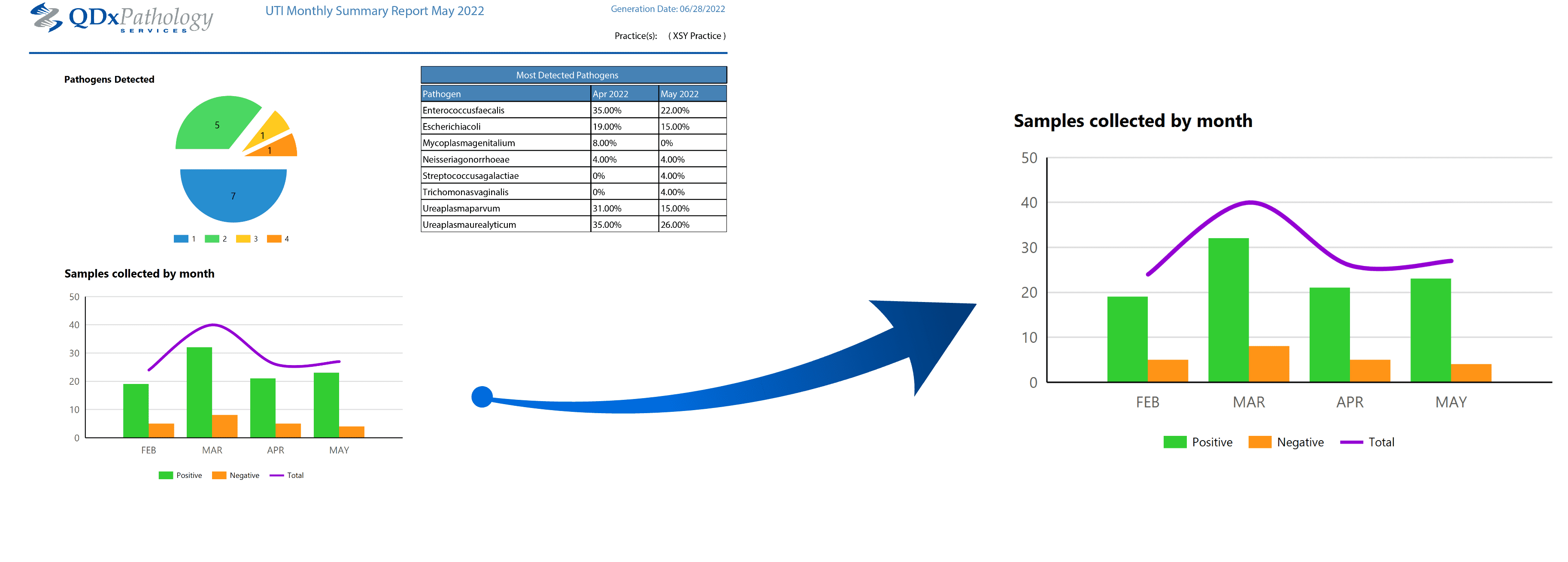
Upon request, QDx provides your practice with clinical management reports that summarize aggregate test result data to support your quality assurance process. Contact your QDx representative to learn more.