Women's Health
Comprehensive Diagnostic Pathology and Pathogen Detection


Diagnostic Support for Women’s Health Providers
QDx pathologists are your diagnostic partners in the diagnosis, prognosis and monitoring of gynecologic pathology including breast, hysterectomies, POC, biopsies, as well as sexual health – including sexually transmitted infections (STIs) testing for both women and men.
QDx pathologists provide pathology examinations and interpretations of GYN tissue samples utilizing special stains and select IHC stains. If necessary, our pathologists provide personalized consultations to the referring provider.
GYN Anatomic Pathology Consultations:
- Hysterectomy
- Uterine
- Products of Conception
- Cervicovaginal
- Endometrial
- FNA
QDx offers the Hologic Thin Prep PAP test collection system which enables multiple testing options from a single vial including PAP, HPV and CT/GC. QDx recommends co-testing, PAP + HPV, as the primary screening method for cervical cancer.
Turn Around Time – 72 hours
CERVICAL SCREENING MENU
PAP, Liquid Based
PAP with HPV Co-testing
PAP + HPV + CT/GC
PAP with HPV reflex – ASCUS
PAP with HPV reflex – ASCUS + CT/GC
PAP Test – Thin Prep
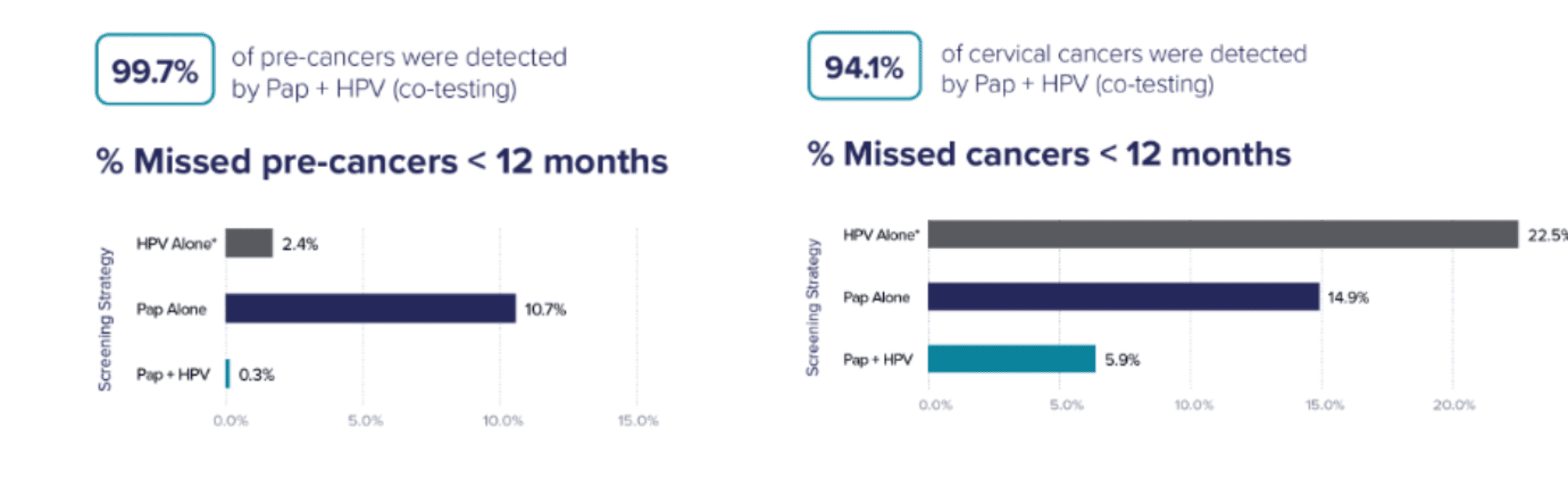
Recent publications representative of US clinical practice showed Pap + HPV misses the fewest cancers and precursors to cancer.
Cervical Screening Menu
PAP, Liquid Based
- Thin Prep PAP
PAP with HPV Co-testing
- Thin Prep PAP
- HPV DNA Genotype 16,18/45
PAP with HPV Co-testing + CT/NG
Thin Prep PAP
HPV DNA Genotype 16,18/45
Chlamydia trachomatis
Neisseria gonorrhoeae
PAP with HPV Reflex if ASC-US/LISL
Thin Prep PAP
If ASC-US/LISL, reflex to HPV DNA Genotype 16,18/45
PAP with HPV Reflex if ASC-US/LISL + CT/NG
Thin Prep PAP
If ASC-US/LISL, reflex to HPV DNA Genotype 16,18/45
Chlamydia trachomatis
Neisseria gonorrhoeae
The QDx vaginitis testing options detect the three most common causes of infectious vaginitis – bacterial vaginosis, candida vaginitis and trichomoniasis.¹ All our vaginitis assays offer the convenience of a single swab collection and the accuracy of PCR testing methodology. Test panels are configured for a targeted approach to utilizing clinically appropriate tests.
VAGINAL HEALTH TEST MENU
- Aptima® Bacterial Vaginosis
- Aptima® Bacterial Vaginosis Expanded
- BD Affirm™ Vaginitis
- Candida & Trichomonas Assay
- Mycoplasma genitalium
- Trichomonas vaginalis
Source: 1. https://www.nichd.nih.gov/health/topics/vaginitis/conditioninfo/causes
Bacterial Vaginosis Panels
- Lactobacillus species
- Gardnerella vaginalis
- Atopobium vaginae
- Megasphaera type 1
- BVAB2
Vaginosis Panel Expanded
- Lactobacillus species
- Gardnerella vaginalis
- Atopobium vaginae
- Megasphaera type 1
- BVAB2
- Chlamydia trachomatis
- Neisseria gonorrhoeae
- Trichomonas vaginalis
- Candida genus
- Candida albicans
- Candida dubliniensis
- Candida parapsilosis
- Candida tropicalis
- Candida glabrata
- Candida krusei
- Lactobacillus species
- Gardnerella vaginalis
- Atopobium vaginae
- Megasphaera type 1
- BVAB2
- Chlamydia trachomatis
- Neisseria gonorrhoeae
- Trichomonas vaginalis
- Candida genus
- Candida albicans
- Candida dubliniensis
- Candida parapsilosis
- Candida tropicalis
- Candida glabrata
- Candida krusei
Vaginal Health Test Details
Aptima® Bacterial Vaginosis
Bacterial Vaginosis (BV) [condition] detection of: Atopobium vaginae, Gardnerella vaginalis, and Lactobacillus species (L. gasseri, L. crispatus, and L. jenseni); no differentiation).BV positive result given when A. vaginae or G. vaginalis are detected and/or Lactobacillus is not detected.
TAT: 3-4 Days
Aptima® Bacterial Vaginosis Expanded
- Bacterial Vaginosis (BV) [condition] detection of: Atopobium vaginae, Gardnerella vaginalis, and Lactobacillus species (L. gasseri, L. crispatus, and L. jenseni); no differentiation).BV positive result given when A. vaginae or G. vaginalis are detected and/or Lactobacillus is not detected.
Candida species detection of: C. albicans, C. glabrata, C. kefyr C. krusei C. parapsilosis and C. tropicalis; no differentiation)
Candida glabrata
Herpes Simplex 1 (HSV1)
Herpes Simplex 2 (HSV2)
Mycoplasma genitalium
Trichomonas vaginalis
TAT: 3-4 Days
BD Affirm™ Vaginitis
- Candida species (detection of: C. albicans, C. glabrata, C. kefyr, C. krusei, C. parapsilosis, and C. tropicalis; no differentiation)
- Gardnerella vaginalis
- Trichomonas vaginalis
TAT: 2-4 Days
Candida & Trichomonas Assay
- Candida species detection of: C. albicans, C. glabrata, C. kefyr C. krusei C. parapsilosis and C. tropicalis; no differentiation)
- Candida glabrata
- Trichomonas vaginalis
TAT: 2-4 Days
Mycoplasma genitalium
TAT: 2-4 Days
Trichomonas vaginalis
TAT: 2-4 Days
Aerobic Vaginosis Panel
- Group B streptococcus
- Staphylococcus aureus
- Escherichia coli
- Enterococcus faecalis
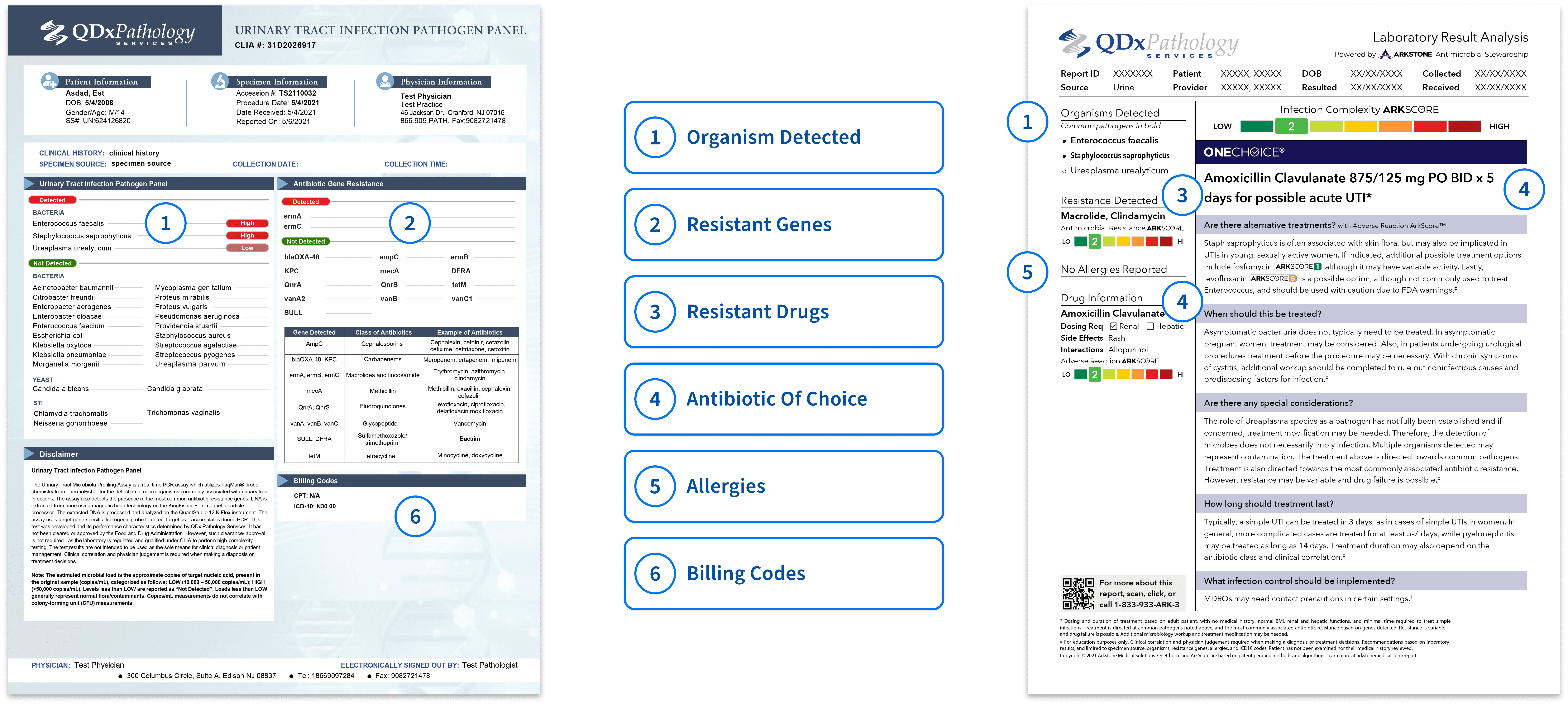
QDx UTI pathogen panel is a PCR assay for the detection of microorganisms commonly associated with urinary tract infections. The assay also detects the presence of the most common antibiotic resistant genes.
- Turn Around Time: 24-48 hours
PATHOGENS
ANTIBIOTIC RESISTANT REPORTING
UTI PATHOGEN PANEL REPORT
The UTI Pathogen panel report is presented in an easy-to-read format with color keys indicating if any pathogen resistant genes are detected and provides the recommended antibiotic.
Pathogens
Bacteria
Morganella morganii
Ureaplasma aparvum
Acinetobacter Baumannii
Mycoplasma genitalium
Ureaplasma urealyticum
Citrobacter freundii
Proteus mirabillis
Enterobacter cloacae
Providencia stuartii
Enterobacter aerogenes
Streptococcus agalactiae
Proteus vulgaris
Enterococcus faecalis
Pseudomonas aeruginosa
Enterococcus faecium
Staphylococcus aureus
Escherichia coli
Staphylococcus saprophyticus
Klebsiella oxytoca
Streptococcus pyogenes
Klebsiella pneumoniae
Yeast
Candida Albicans
Candida glabrata
STI
Chlamydia trachomatis
Neisseria gonorrhoeae
Trichomonas vaginalis
Antibiotic Resistant Gene Key
| Gene Detected | Class of Antibiotics | Example of Antibiotics |
|---|---|---|
| AmpC | Cephalosporins | Cephalexin, cefdinir, cefazolin cefixime, ceftriaxone, cefoxitin |
| blaOXA-48, KPC | Carbapenems | Meropenem, ertapenem, imipenem |
| ErmA, ErmB, ErmC | Macrolides and lincosamide | Erythromycin, azithromycin, clindamycin |
| mecA | Methicillin | Methicillin, oxacillin, cephalexin, cefazolin |
| QnrA, QnrS | Fluoroquinolones | Levofloxacin, ciprofloxacin, delafloxacin moxifloxacin |
| VanA, VanB, VanC | Glycopeptide | Vancomycin |
| SULL, DFRA | Sulfamethoxazole/ trimethoprim | Bactrim |
| tetM | Tetracycline | Minocycline, doxycycline |
AmpC
Class of Antibiotics:
Cephalosporins
Example of Antibiotics:
Cephalexin, cefdinir, cefazolin cefixime, ceftriaxone, cefoxitin
blaOXA-48, KPC
Class of Antibiotics:
Carbapenems
Example of Antibiotics:
Meropenem, ertapenem, imipenem
ErmA, ErmB, ErmC
Class of Antibiotics:
Macrolides and lincosamide
Example of Antibiotics:
Erythromycin, azithromycin, clindamycin
mecA
Class of Antibiotics:
Methicillin
Example of Antibiotics:
Methicillin, oxacillin, cephalexin, cefazolin
QnrA, QnrS
Class of Antibiotics:
Fluoroquinolones
Example of Antibiotics:
Levofloxacin, ciprofloxacin, delafloxacin moxifloxacin
VanA, VanB, VanC
Class of Antibiotics:
Glycopeptide
Example of Antibiotics:
Vancomycin
SULL, DFRA
Class of Antibiotics:
Sulfamethoxazole/ trimethoprim
Example of Antibiotics:
Bactrim
tetM
Class of Antibiotics:
Tetracycline
Example of Antibiotics:
Minocycline, doxycycline
SEXUALLY TRANSMITTED INFECTIONS (STIs)
According to the CDC, 1 in 5 people in the US have an STI. ¹ New infections, both diagnosed and undiagnosed, are estimated at 13M for human papillomavirus (HPV), 6.9M for Trichomoniasis, 4M for Chlamydia, 1.6M for Gonorrhea, and 572K for herpes simplex virus type 2 (HSV-2).¹ Chlamydia, trichomoniasis, genital herpes, and HPV accounted for 98% of all prevalent STIs and 93% of all new STIs in 2018.¹ For guidance, the CDC’s updated 2021 STI screening recommendations may be found on their website. QDx Pathology uses nucleic acid amplification (NAA)-based assays to detect and differentiate pathogen RNA, allowing for rapid and reliable test results.
HPV Testing
HPV w/ Genotype 16,18,45
- HPV DNA Genotyping 16,18,45
STI Test Details
CT/NG
Chlamydia trachomatis
Neisseria gonorrhoeae
TAT: 3-4 Days
HPV with Reflex to Genotyping for 16, 18/45 if HPV+
Tests for high-risk human papillomavirus (HPV) types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) without differentiation and if positive, reflexes to genotyping for HPV types 16 and 18,45; type 18 cannot be differentiated from type 45.
TAT: 3-4 Days
HSV 1&2
Herpes Simplex Virus 1 (HSV1)
Herpes Simplex Virus 2 (HSV2)
TAT: 3-4 Days
Mycoplasma genitalium
TAT: 2-4 Days
Trichomonas vaginalis
TAT: 2-4 Days
STI TEST MENU
CT/NG
HPV with Reflex to Genotyping for 16, 18/45 if HPV+
HSV 1&2
Mycoplasma Genitalium
Trichomonas Vaginalis
Source: 1. https://www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm